Photochemical intramolecular cyclization of o-alkynylaryl isocyanides with organic dichalcogenides leading to 2,4-bischalcogenated quinolines†
Abstract
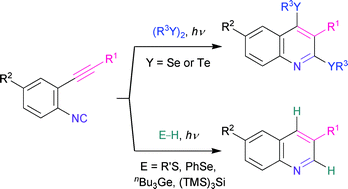
When a mixture of o-alkynylaryl isocyanides and organic dichalcogenides such as diselenides or ditellurides was irradiated with light of wavelength over 300 or 400 nm, the intramolecular cyclization of the isocyanides took place to afford the corresponding 2,4-bischalcogenated quinolines selectively. The photochemical cyclization of 2-(phenylethynyl)phenyl isocyanide could also proceed in the presence of hydrogen transfer reagents such as tris(trimethylsilyl)silane, tributylgermyl hydride, alkanethiols, and benzeneselenol, providing the corresponding 3-phenylquinoline as the result of 2,4-dihydrogenation.
- This article is part of the themed collection: Free Radical Chemistry special themed issue in memory of Athel Beckwith

 Please wait while we load your content...
Please wait while we load your content...