Kai Jiang, Ziyan Zhao, Xiaodong Yin, Fangjun Chen and Biaolin Yin
Org. Chem. Front., 2025,12, 1579-1585
DOI:
10.1039/D4QO02173H,
Research Article
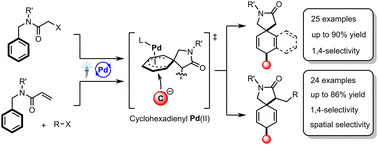
Excited-palladium catalysis has already been exploited to achieve 1,4-difunctionalization of dienes via the radical generation of an allylpalladium intermediate. Herein, nonactivated phenyl rings, which can be treated as masked trienes, have accomplished dearomative 1,4-dicarbofunctionalization in an excited-palladium catalyzed two- or three-component reaction system. A wide range of alkyl bromides and 1,3-dicarbonyl compounds, playing the roles of radical precursors and nucleophiles, respectively, were found to be suitable for this reaction. Various three-dimensional molecular architectures with multiple quaternary carbon centers were efficiently constructed by means of this mild reaction.