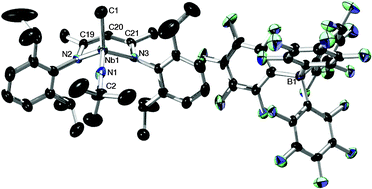
The synthesis and reactivity of the cationic niobium and tantalum monomethyl complexes [(BDI)MeM(NtBu)][X] (BDI = [Ar]NC(CH3)CHC(CH3)N[Ar], Ar = 2,6-iPr2C6H3; M = Nb, Ta; X = MeB(C6F5)3, B(C6F5)4] was investigated. The cationic alkyl complexes failed to irreversibly bind CO but formed phosphine-trapped acyl complexes [(BDI)(R3PC(O)Me)M(NtBu)][B(C6F5)4] (R = Et, Cy) in the presence of a combination of trialkylphosphines and CO. Treatment of the monoalkyl cationic Nb complex with XylNC (Xyl = 2,6-Me2-C6H3) resulted in irreversible formation of the iminoacyl complex [(BDI)(XylN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(Me))Nb(NtBu)][B(C6F5)4], which did not bind phosphines but would add a methide group to the iminoacyl carbon to provide the known ketimine complex (BDI)(XylNCMe2)Nb(NtBu). Further stoichiometric chemistry explored i) migratory insertion reactions to form new alkoxide, amidinate, and ketimide complexes; ii) protonolysis reactions with Ph3SiOH to form thermally robust cationic siloxide complexes; and iii) catalytic high-density polyethylene formation mediated by the cationic Nb methyl complex.
C(Me))Nb(NtBu)][B(C6F5)4], which did not bind phosphines but would add a methide group to the iminoacyl carbon to provide the known ketimine complex (BDI)(XylNCMe2)Nb(NtBu). Further stoichiometric chemistry explored i) migratory insertion reactions to form new alkoxide, amidinate, and ketimide complexes; ii) protonolysis reactions with Ph3SiOH to form thermally robust cationic siloxide complexes; and iii) catalytic high-density polyethylene formation mediated by the cationic Nb methyl complex.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(Me))Nb(NtBu)][B(C6F5)4], which did not bind
C(Me))Nb(NtBu)][B(C6F5)4], which did not bind 

 Please wait while we load your content...
Please wait while we load your content...