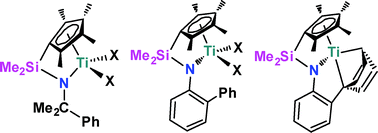
The synthesis of the proligands C5Me4HSiMe2N(H)R) (R = CMe2Ph 1, 2-C6H4Ph 2) was accomplished via a straightforward salt metathesis reaction of the appropriate lithium amide and ClSiMe2(C5Me5H). Generation of the dilithio salt and reaction with TiCl3·(THF)3 followed by oxidation gave C5Me4SiMe2N(C6H4Ph)TiCl2 (3) in low yield. In contrast, deprotonation of 1 and 2 and reaction with (Me2N)2TiCl2 afforded C5Me4(SiMe2NR)Ti(NMe2)2 (R = CMe2Ph 4, 2-C6H4Ph 5), respectively, in good yields Treatment with MeI gave the analogs C5Me4(SiMe2NR)TiI2 (R = CMe2Ph 6, 2-C6H4Ph 7). Reduction of 7 with potassium graphite afforded C5Me4(SiMe2NC6H4Ph)Ti 8. Treatment of 6 and 7 with MeMgBr afforded C5Me4(SiMe2NR)TiMe2 (R = CMe2Ph 9, 2-C6H4Ph 10). Complexes 9 and 10 in combination with the activator [Ph3C][B(C6F5)4] catalyzed the polymerization of styrene and ethylene. Copolymerization was also investigated. While the catalyst derived from 10 showed poor activity, compound 9 showed markedly higher activity than 10 and (C5Me4)SiMe2(NtBu)]TiMe2.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...