Identification of 3,6-di-O-acetyl-1,2,4-O-orthoacetyl-α-d-glucopyranose as a direct evidence for the 4-O-acyl group participation in glycosylation†‡
Abstract
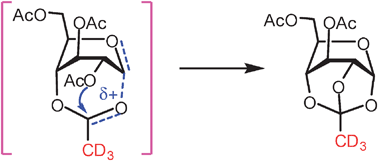
The formation of 3,6-di-O-acetyl-1,2,4-O-orthoacetyl-α-D-glucopyranose was observed in the gold(I)-catalyzed glycosidation of peracetyl glucopyranosyl ortho-hexynylbenzoate; experiments with substrates bearing deuterium labeled 2-O-acetyl or 4-O-acetyl groups indicated that the orthoacetate was derived from the 4-O-acetyl group, which provided a direct evidence for the remote participation of the 4-O-acyl group in
- This article is part of the themed collection: Glycochemistry & glycobiology

 Please wait while we load your content...
Please wait while we load your content...