Electronic structure modulation of bifunctional oxygen catalysts for rechargeable Zn–air batteries†
Abstract
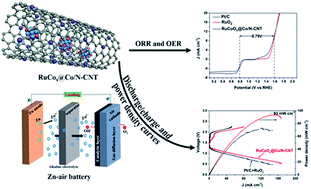
Extensive efforts have been devoted to develop bifunctional oxygen catalysts for rechargeable zinc–air batteries (ZABs) owing to the extremely high specific energy density, low cost and safety of these emerging batteries. The oxygen catalysts play roles in maximizing the energy conversion efficiencies of ZABs. Herein, a strategy of electronic structure modulation is used by ruthenium doping and post-oxidation treatment in cobalt encapsulated N-doped carbon nanotubes for enhancing catalytic activities of the oxygen reduction reaction (ORR) and oxygen evolution reaction (OER). First principles calculations reveal that doping Ru into Co or CoOx enhances charge transfer from Ru to carbon atoms adjacent to the doped N atom and then promotes the reversible oxygen reactions. The achieved catalysts (denoted as RuCoOx@Co/N-CNT) exhibit efficient catalytic activities driving both ORR and OER with a small overpotential gap (EOER − EORR = 0.79 V). Specifically, the ZABs assembled with RuCoOx@Co/N-CNT catalysts display an open-circuit potential of 1.44 V, a specific capacity of 788 mA h g−1, a power density of 93 mW cm−2 and long-term discharge–charge cycling stability, even superior to those of commercial Pt/C and RuO2 electrodes. This work proves that modulating the electronic structure of active sites by doping or post oxidation treatment is an efficient way to improve the catalytic performance.
- This article is part of the themed collection: Journal of Materials Chemistry A HOT Papers


 Please wait while we load your content...
Please wait while we load your content...