Access to valuable building blocks by the regio- and enantioselective ring opening of itaconic anhydride by lipase catalysis†
Abstract
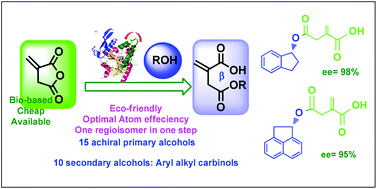
Herein, we report for the first time the highly regio- and enantioselective ring opening of a biobased itaconic anhydride catalyzed by the Pseudomonas cepacia lipase (PCL) in tert-butyl methyl ether (TBME) at room temperature. This method is easy, efficient and eco-friendly and can be performed in one step with a series of highly valuable monoester itaconates (achiral or enantioenriched) using various alcohols as nucleophiles with 100% atom economy. In all cases, the β-monoester isomer was the predominant product of the reaction. Using achiral primary alcohols as substrates, a variety of novel itaconates were obtained in moderate to excellent yields (50–90%). For select examples, product characterization was carried out using X-ray diffraction, in addition to the standard techniques. The application of this approach was performed for the preparation of enantioenriched 4-monoester itaconates via enzymatic kinetic resolution.


 Please wait while we load your content...
Please wait while we load your content...