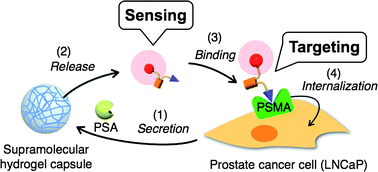
A supramolecular hydrogel 1, prepared at high concentration (5–10 wt%), exhibited mechanical toughness comparable to that of a polymer hydrogel, even though the gel was constructed solely by noncovalent bonding interactions among small molecules. The mechanical toughness and thermal reversibility of gel 1 allowed us to fabricate a supramolecular hydrogel capsule (SH-capsule) 1 which was easily handled and was stable in aqueous and cell culture media. The mechanical and substance-release/uptake properties of SH-capsule 1 were investigated by atomic force microscope (AFM) indentation, fluorescence spectroscopy, and confocal laser scanning microscopy (CLSM). On the basis of those properties we successfully designed an enzyme- and cell-responsive SH-capsule. To install a function of enzyme-responsive substance release into the SH-capsule 1, enzyme-labile, amphiphilic additive 2 was embedded in supramolecular nanofibers of 1 through a supramolecular co-assembly method. As a proof-of-concept, we constructed the functional SH-capsule 1/2 that can release a model fluorescent drug triggered by prostate specific antigen (PSA)-catalyzed proteolysis. Selective release of the fluorescent substance was exploited to both assay PSA activity and detect prostate cancer (PCa) cells. We also clearly demonstrated that the released fluorescent substance was delivered and internalized into the PCa cells, mediated by binding to the membrane-bound protein prostate-specific membrane antigen (PSMA), which is over-expressed on a plasma membrane of the PCa cells.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...