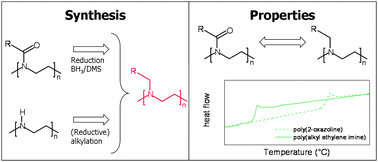
The synthesis of a series of poly(alkyl ethylene imine) (PalkylEI) with varying side chain lengths of 1, 2, 3 and 10 carbon atoms as well as a benzyl side group was performed. A series of polymers was synthesized from a direct reduction reaction of the corresponding poly(2-oxazoline) while another series was prepared by (reductive) alkylation of linear poly(ethylene imine) (PEI). The thermal and solubility properties of the resulting polymers were investigated and compared to the starting poly(2-oxazoline)s in order to study the effects of removing the carbonyl from the polymer structure. 1H NMR spectroscopy revealed quantitative formation of the PalkylEI, i.e. no residual signals of the poly(2-oxazoline) or linear PEI were observed. Thermal investigations revealed that the thermal transitions, melting and glass transition temperatures, significantly decreased after decarboxylation due to an increase in chain flexibility. Furthermore, solubility measurements indicated that the PalkylEIs are more hydrophobic compared to the corresponding poly(2-oxazoline)s, whereby poly(ethyl ethylene imine) revealed pH and temperature responsive solubility behavior in water.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...