Highly efficient and enantioselective biotransformations of β-lactam carbonitriles and carboxamides and their synthetic applications†‡
Abstract
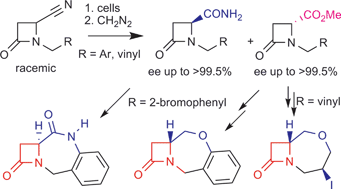
Catalyzed by Rhodococcus erythropolis AJ270, a
- This article is part of the themed collection: Chemical Biology

 Please wait while we load your content...
Please wait while we load your content...