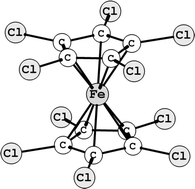
The molecular structure, equilibrium conformation and barrier to internal rotation in decachloroferrocene, Fe(η-C5Cl5)2, determined by gas electron diffraction†
Abstract
The molecular structure of
- This article is part of the themed collection: Collection of articles dedicated to Professor David W. H. Rankin on the occasion of his retirement

 Please wait while we load your content...
Please wait while we load your content...