In situ preparation of a La1.2Sr0.8Mn0.4Fe0.6O4 Ruddlesden–Popper phase with exsolved Fe nanoparticles as an anode for SOFCs†
Abstract
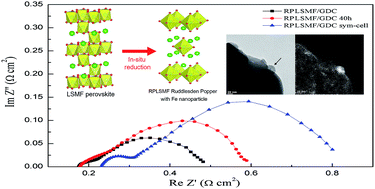
A highly stable electrode material of Ruddlesden–Popper structure, La1.2Sr0.8Mn0.4Fe0.6O4 (RPLSMF), is prepared from La0.6Sr0.4Mn0.2Fe0.8O3 (LSMF) perovskite by in situ annealing in flowing H2 at the operation temperature of solid oxide fuel cells. The crystallinity, morphology, and oxidation states of each element and electrochemical properties of RPLSMF are characterized. Doping Mn into the B-site of RPLSMF improves the phase stability of the structure in H2 to prevent formation of La2O3. The XPS results also suggest that improved phase stability promotes formation of Fe2+/3+ pairs that facilitate fuel oxidation by redox coupling. Additionally, during phase transition to RPLSMF, metallic Fe nanoparticles form, which enlarge H2 chemisorption and oxidation sites. Consequently, RPLSMF exhibits outstanding and stable electrochemical activity with a maximum power density of 0.72 W cm−2 at 1073 K when used as an anode material in LSGM electrolyte-supported cells. As the phase transition between the RPLSMF and LSMF is reversible under a redox environment, RPLSMF/GDC is applied as an electrode in the symmetrical cell of RPLSMF-GDC/LSGM/LSMF-GDC. It exhibits a substantial power density of 0.64 W cm−2 with a total polarization resistance of 0.51 Ω cm2.


 Please wait while we load your content...
Please wait while we load your content...