Production of biodiesel: kinetics and reusability studies of the Mg–Al hydrotalcite catalyst using Jatropha oil
Abstract
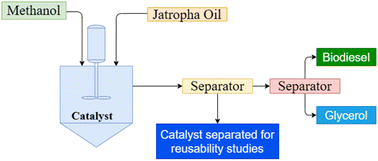
This study focuses on the use of the co-precipitation method to prepare Mg–Al layered double hydroxide (LDH) catalysts for the transesterification conversion of Jatropha oil into biodiesel. X-Ray diffraction (XRD), scanning electron microscopy (SEM), and BET methods were used to characterize the crystallinity, morphology, and surface area of the catalyst. The effects of varying reaction conditions, such as time, catalyst loading, reaction temperature, and methanol to oil molar ratio, on biodiesel yield, were investigated. The optimal experimental conditions for achieving a 95 percent yield were identified as a temperature of approximately 70 °C, a catalyst loading of 3 percent by weight, and a molar ratio of methanol to Jatropha oil of 12 to 1. Catalyst regeneration was also studied to determine reusability, and the activation energy was evaluated to be around 26.7 kJ mol−1. The results showed that the catalyst could be reused for up to 5 cycles with a yield reduction of less than ∼10%. The study demonstrates the potential of using inexpensive Mg–Al LDH catalysts for large-scale biodiesel production.


 Please wait while we load your content...
Please wait while we load your content...