Dynamic behavior of an artificial protein needle contacting a membrane observed by high-speed atomic force microscopy†
Abstract
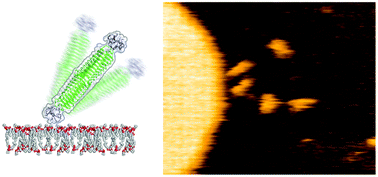
Bacteriophage T4 and other bacteriophages have a protein component known as a molecular needle which is used for the transmembrane reaction in the infection process. In this paper, the transmembrane reaction mechanisms of artificial protein needles (PNs) constructed by protein engineering of the component protein of bacteriophage T4 are elucidated by observation of single-molecules by high-speed atomic force microscopy (HS-AFM) and molecular dynamics (MD) simulations. The HS-AFM images indicate that the tip of the needle structure stabilizes the interaction of the needle with the membrane surface and is involved in controlling the contact angle and angular velocity with respect to the membrane. The MD simulations indicate that the dynamic behavior of PN is governed by hydrogen bonds between the membrane phosphate fragments and the tip. Moreover, quartz crystal microbalance (QCM) and electrophysiological experiments indicate that the tip structure of PN affects its kinetic behavior and membrane potential. These results demonstrate that protein assemblies derived from natural biosupramolecules can be used to create nanomaterials with rationally-designed functionality.


 Please wait while we load your content...
Please wait while we load your content...