Self-recovery in Li-metal hybrid lithium-ion batteries via WO3 reduction†
Abstract
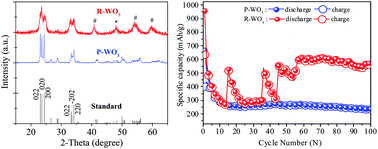
It has been a challenge to use transition metal oxides as anode materials in Li-ion batteries due to their low electronic conductivity, poor rate capability and large volume change during charge/discharge processes. Here, we present the first demonstration of a unique self-recovery of capacity in transition metal oxide anodes. This was achieved by reducing tungsten trioxide (WO3) via the incorporation of urea, followed by annealing in a nitrogen environment. The reduced WO3 successfully self-retained the Li-ion cell capacity after undergoing a sharp decrease upon cycling. Significantly, the reduced WO3 also exhibited excellent rate capability. The reduced WO3 exhibited an interesting cycling phenomenon where the capacity was significantly self-recovered after an initial sharp decrease. The quick self-recoveries of 193.21%, 179.19% and 166.38% for the reduced WO3 were observed at the 15th (521.59/457.41 mA h g−1), 36th (538.49/536.61 mA h g−1) and 45th (555.39/555.39 mA h g−1) cycles respectively compared to their respective preceding discharge capacity. This unique self-recovery phenomenon can be attributed to the lithium plating and conversion reaction which might be due to the activation of oxygen vacancies that act as defects which make the WO3 electrode more electrochemically reactive with cycling. The reduced WO3 exhibited a superior electrochemical performance with 959.1/638.9 mA h g−1 (1st cycle) and 558.68/550.23 mA h g−1 (100th cycle) vs. pristine WO3 with 670.16/403.79 mA h g−1 (1st cycle) and 236.53/234.39 mA h g−1 (100th cycle) at a current density of 100 mA g−1.


 Please wait while we load your content...
Please wait while we load your content...
