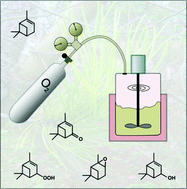
The liquid-phase oxidation of the renewable olefin α-pinene with molecular oxygen yields several valuable compounds for the fine-chemical industry. The most important products are verbenol/-one and α-pinene oxide. Following our previous work on the radical autoxidation at atmospheric pressure, this contribution addresses the influence of the oxygen pressure on the reaction mechanism and the product distribution. Trapping of the radical epoxide-precursor by O2 causes a decrease of the epoxide selectivity, as well as the formation of a thermally unstable dialkylperoxide. This dialkylperoxide accelerates the rate significantly, due to an enhancement of the radical initiation. Although this causes a decrease of the radical chain-length, the amount of products produced in the chain-termination can still be neglected compared to the amount produced in the chain-propagations. Parallel to this, the ketone to alcohol ratio increases at higher oxygen pressure, due to the reaction of alkoxyl radicals with O2, as well as a reaction of O2 with the addition product of the alkoxyl radicals and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond of the substrate. For O2 partial pressures of 1 to 80 bar, rate constants of important reactions are extracted from the experimental observations via differential modelling, and confronted with literature values and/or quantum-chemical predictions. The derived mechanism is supported at the molecular level and provides a reliable description of the experimental observations.
C double bond of the substrate. For O2 partial pressures of 1 to 80 bar, rate constants of important reactions are extracted from the experimental observations via differential modelling, and confronted with literature values and/or quantum-chemical predictions. The derived mechanism is supported at the molecular level and provides a reliable description of the experimental observations.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond of the substrate. For O2 partial pressures of 1 to 80 bar, rate constants of important reactions are extracted from the experimental observations via differential modelling, and confronted with literature values and/or quantum-chemical predictions. The derived mechanism is supported at the molecular level and provides a reliable description of the experimental observations.
C double bond of the substrate. For O2 partial pressures of 1 to 80 bar, rate constants of important reactions are extracted from the experimental observations via differential modelling, and confronted with literature values and/or quantum-chemical predictions. The derived mechanism is supported at the molecular level and provides a reliable description of the experimental observations.

 Please wait while we load your content...
Please wait while we load your content...