Enhanced degradation of norfloxacin by Ce-mediated Fe-MIL-101: catalytic mechanism, degradation pathways, and potential applications in wastewater treatment†
Abstract
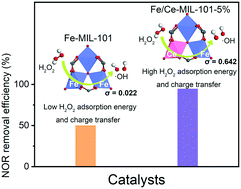
Novel Ce-mediated Fe-MIL-101 (Fe/Ce-MIL-101) Fenton catalysts were synthesized to efficiently remove norfloxacin (NOR). The substitution of Ce remarkably enhanced the catalytic performance of Fe-MIL-101, and the degradation efficiency of NOR (10 mg L−1) increased from 50.1% to 94.8% within 60 min. NOR could be completely degraded by Fe/Ce-MIL-101 after 180 min. Moreover, Fe/Ce-MIL-101 showed excellent stability and reusability in simulated and actual wastewater samples. Density functional theory (DFT) calculations revealed that the introduction of Ce(III) and formation of hetero-coordinated Fe–O–Ce moieties in the secondary building units (SBUs) of Fe-MIL-101 could induce electronic distribution asymmetry, benefit the interaction between the metal sites and H2O2, accelerate electron transfer, and further facilitate H2O2 activation. The degradation pathways of NOR were identified based on liquid chromatography-mass spectrometry (LC-MS) analysis and Fukui function calculation. Nine benzene ring-containing intermediates were initially detected after 20 min of degradation, and only two byproducts (“harmless” category) were preserved after 180 min. The opening of the piperazine ring and transformation of the quinolone group were mainly caused by the formed hydroxyl free radicals (˙OH). This work demonstrates a new strategy to enhance the performance of Fe-based Fenton-like catalysts by mediating the asymmetrical electronic distribution of coordinated Fe sites.


 Please wait while we load your content...
Please wait while we load your content...