Chemical extraction of Zn from ZnMn2O4-based spinels
Abstract
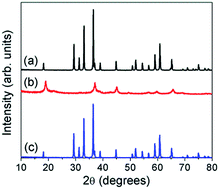
With an aim to increase the energy density compared to current Li-ion batteries, there is immense interest in rechargeable batteries with multivalent cations, such as Mg2+ and Zn2+, as they could provide higher charge storage capacity. α-MnO2 has been investigated as a cathode for Zn-ion batteries, but other structures could be explored. Accordingly, this study investigates the chemical extraction of Zn from the spinel compositions ZnMn2−xNixO4 (x = 0, 0.5, and 1) using H2SO4 and NO2BF4. Acid treatment is able to extract Zn from the structure through a Mn3+ disproportionation reaction that is dependent on the Mn3+ content. Zn extraction decreases with increasing Ni content due to the decrease in Mn3+ content; in fact, ZnMnNiO4 does not lose any Zn upon acid treatment because all of the Mn ions are in the 4+ state. Treatment with NO2BF4, however, is unable to extract any Zn from the samples, unlike in the analogous LiMn2O4 spinel. This is believed to be due to the high electrostatic repulsion that Zn2+ ions would feel from other Zn2+ ions in neighboring tetrahedral sites as they have to diffuse through the unoccupied octahedral sites in the spinel lattice, and Zn2+ does not prefer octahedral sites. Because NO2BF4 more closely approximates the extraction mechanism experienced in a Zn-ion cell, it appears that the ideal spinel structure is not suitable as a Zn-ion battery cathode unless synthesis conditions or additional treatments were used to create vacancies in the structure that allows for easier Zn-ion diffusion without much electrostatic repulsion.

 Please wait while we load your content...
Please wait while we load your content...