Effects of electron-donating groups on the photocatalytic reaction of MOFs†
Abstract
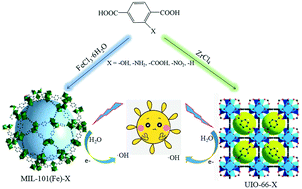
Regulating the synthesis of photocatalytic materials at the molecular level could affect the absorption of light and guide the synthesis of highly efficient photocatalysts for the photocatalytic degradation organic pollutants. The results of UV-visible diffuse reflectance spectroscopy (UV-vis DRS), zeta potential and the photocatalytic degradation of phenanthrene in water both proved that the stronger the electron-donating groups in MIL-101(Fe)-X and UIO-66-X (X = –OH, –NH2, –COOH, –NO2 and –H), the better the photocatalytic efficiency, and the catalytic efficiency followed the order of –OH > –NH2 > –COOH > –NO2 > –H. This order was due the electronegativity regulation of the MIL-101(Fe)-X and UIO-66-X by the electron-donating groups in their structure. The XRD, UV-vis DRS and XPS studies of MIL-101(Fe)-X and UIO-66-X showed that the structure of these two series of MOFs remained unchanged after the photocatalytic degradation of phenanthrene. The above results provide a direction for the synthesis of highly efficient photocatalysts.


 Please wait while we load your content...
Please wait while we load your content...