Synthesis and properties of organic/inorganic hybrid branched-graft copolymers and their application to solid-state electrolytes for high-temperature lithium-ion batteries†
Abstract
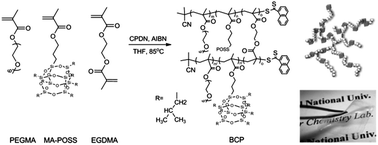
A series of organic/inorganic hybrid branched (BCPs) and linear (LCPs)-graft copolymers comprising poly(ethylene glycol) methyl ether methacrylate (PEGMA), 3-(3,5,7,9,11,13,15-heptaisobutyl-pentacyclo[9.5.1.13,9.15,15.17,13]octasiloxane-1-yl)propyl methacrylate (MA-POSS), and ethylene glycol dimethacrylate (EGDMA) were synthesized via reversible addition–fragmentation chain transfer (RAFT) polymerization for application to solid polymer electrolyte (SPE) materials in high-temperature lithium-ion batteries. Dimensionally stable free-standing films were obtained when the MA-POSS contents in BCPs and LCPs are larger than 21 mol% and maintained their original shape and storage modulus even if the temperature increases up to 90 °C. The maximum ionic conductivity value of the BCP electrolyte containing 21 mol% of MA-POSS was 1.6 × 10−4 S cm−1 at 60 °C and that of the LCP electrolyte containing 21 mol% of MA-POSS was 5.6 × 10−5 S cm−1 at 60 °C, indicating that the branched-graft copolymer electrolyte has higher ionic conductivity than the linear-graft counterpart, due to the increased chain mobility, as estimated by a smaller Tg value. All-solid-state batteries prepared using the BCP electrolyte showed a reasonable cell performance at 60 °C without causing safety problems, demonstrating great potential of BCPs as SPE materials for high-temperature battery systems.

 Please wait while we load your content...
Please wait while we load your content...