Highly fluorescent photochromic diarylethene with an excellent fatigue property†
Abstract
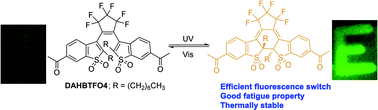
A series of diarylethenes and their disulfonyl derivatives such as 1,2-bis(2-heptyl-1-benzothiophene-3-yl)perfluorocyclopentene (HBTF6), 1,2-bis(6-acetyl-2-heptyl-1-benzothiophene-3-yl)perfluorocyclopentene (DAHBTF6), 1,2-bis(2-heptyl-1-benzothiophene-1,1-dioxide-3-yl)perfluorocyclopentene (HBTFO4), and 1,2-bis(6-acetyl-2-heptyl-1-benzothiophene-1,1-dioxide-3-yl)perfluorocyclopentene (DAHBTFO4), have been prepared for the examination of a substituent and an oxidation effect on photochromic and photophysical properties. Steady state fluorescence studies indicated that the disulfonyl diarylethenes were highly fluorescent only in the closed form. The introduction of acetyl groups at the 6,6′ positions and heptyl groups at the 2,2′ positions in disulfonyl diarylethene gave rise to an increase in the fluorescence quantum yield and fluorescence lifetime. Fatigue properties of oxidized diarylethenes were improved by the introduction of acetyl groups, indicating that the photochromic properties of diarylethene are strongly affected by substituents. We also demonstrated the recording to and erasing of information from the materials by an alternating illumination with UV and visible light, and the accompanying strong fluorescence intensity changes provided a highly efficient information readout system, which may be applicable to erasable optical data-storage elements.

 Please wait while we load your content...
Please wait while we load your content...