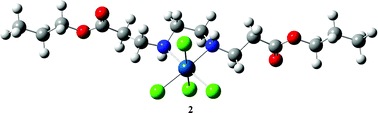
The studies on synthetic, spectroscopic and biological properties of platinum(IV) complexes, [PtCl4(R2eddp)] (R = Et, 1; n-Pr, 2), containing κ2N,N′ bidentate ligands, esters of ethylenediamine-N,N′-di-3-propionic acid (HOOCCH2CH2NHCH2CH2NHCH2CH2COOH, H2eddp), are reported. Complexes have been characterized by infrared, 1H and 13C NMR spectroscopy and elemental analysis and it was concluded that the coordination of the ligands occurs via nitrogen donor atoms of the ester ligands (R2eddp). Cytotoxicity studies were performed for ligand precursors and corresponding platinum(IV) complexes. Although the n-Pr2eddp·2HCl itself showed no activity (IC50 values > 125 μM) in selected cell lines, the activity of complex 2, via apoptotic mode of cell death, has increased significantly for a broad range of cancer cell lines tested in vitro (IC50 = 8.6–49 μM). As it was found that complexes 1 and 2 are able to interact with pBR322 plasmid DNA, platinum(IV) complexes of this type may act as drugs and pro-drugs.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...