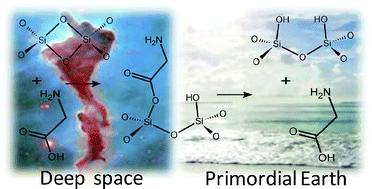
The reaction of glycine (Gly) with a strained (SiO)2 four-membered ring defect (D2) at the surface of an interstellar silica grain dust has been studied at ONIOM2[B3LYP/6-31+G(d,p):MNDO] level within a cluster approach in the context of hypothetical reactions occurring in the interstellar medium. The D2 opens up exothermically for reaction with Gly (ΔrU0=−26.3 kcal mol−1) to give a surface mixed anhydride Ssurf–O–C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–CH2NH2 as a product. The reaction barriers, ΔU≠0, are 0.1 and 10.4 kcal mol−1 for reactive channels involving COOH and NH2 as attacking groups, respectively. Calculations show the surface mixed anhydride to be rather stable under the action of interstellar processes, such as reactions with isolated H2O and NH3 molecules or the exposure to cosmic rays and UV radiation. The hydrolysis of the surface mixed anhydride to release again Gly was modelled by microsolvation (from 1 to 4 H2O molecules) mimicking what could have happened to the interstellar dust after seeding the primordial ocean in the early Earth. Results for these calculations show that the reaction is exergonic and activated, the ΔrG298 becoming more negative and the ΔG≠298 being dramatically reduced as a function of increasing number of H2O molecules. The present results are relevant because they show that defects present at interstellar dust surfaces could have played a significant role in capturing, protecting and delivering essential prebiotic compounds on the early Earth.
O)–CH2NH2 as a product. The reaction barriers, ΔU≠0, are 0.1 and 10.4 kcal mol−1 for reactive channels involving COOH and NH2 as attacking groups, respectively. Calculations show the surface mixed anhydride to be rather stable under the action of interstellar processes, such as reactions with isolated H2O and NH3 molecules or the exposure to cosmic rays and UV radiation. The hydrolysis of the surface mixed anhydride to release again Gly was modelled by microsolvation (from 1 to 4 H2O molecules) mimicking what could have happened to the interstellar dust after seeding the primordial ocean in the early Earth. Results for these calculations show that the reaction is exergonic and activated, the ΔrG298 becoming more negative and the ΔG≠298 being dramatically reduced as a function of increasing number of H2O molecules. The present results are relevant because they show that defects present at interstellar dust surfaces could have played a significant role in capturing, protecting and delivering essential prebiotic compounds on the early Earth.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–CH2NH2 as a product. The reaction barriers, ΔU≠0, are 0.1 and 10.4 kcal mol−1 for reactive channels involving COOH and NH2 as attacking groups, respectively. Calculations show the surface mixed anhydride to be rather stable under the action of interstellar processes, such as reactions with isolated H2O and
O)–CH2NH2 as a product. The reaction barriers, ΔU≠0, are 0.1 and 10.4 kcal mol−1 for reactive channels involving COOH and NH2 as attacking groups, respectively. Calculations show the surface mixed anhydride to be rather stable under the action of interstellar processes, such as reactions with isolated H2O and 

 Please wait while we load your content...
Please wait while we load your content...