Single Cu–N4 sites enable atomic Fe clusters with high-performance oxygen reduction reactions†
Abstract
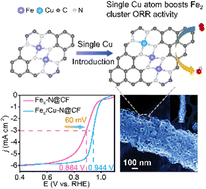
Atomically dispersed Fe–N4 catalysts have proven to be promising alternatives to commercial Pt/C for the oxygen reduction reaction. Most reported Fe–N4 catalysts suffer from inferior O–O bond-breaking capabilities due to superoxo-like O2 adsorption, though the isolated dual-atomic metal site strategy is extensively adopted. Atomic Fe clusters show greater promise for promoting O–O bond cleavage by undergoing peroxo-like O2 adsorption. However, the excessively strong binding strength between Fe clusters and oxygenated intermediates sacrifices the activity. Here, we first report a Fex/Cu–N@CF catalyst with atomic Fe clusters functionalized by adjacent single Cu–N4 sites anchored on a porous carbon nanofiber membrane. Theoretical calculation indicates that the single Cu–N4 sites can modulate the electronic configuration of Fe clusters to reduce the O2* protonation reaction free energy which ultimately enhances the electrocatalytic performance. In particular, the Cu–N4 sites can increase the overlap between the d orbitals of Fe and p orbitals of O to accelerate O–O cleavage in OOH*. As a result, this unique atomic catalyst exhibits a half potential (E1/2) of 0.944 V in alkaline medium, exceeding that of commercial Pt/C, whereas its acidic performance E1/2 = 0.815 V is comparable to that of Pt/C. This work shows the great potential of single atoms for improvements in atomic cluster catalysts.


 Please wait while we load your content...
Please wait while we load your content...