Abstract
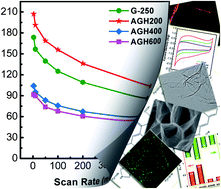
Graphene with mediated surface properties and three-dimensional hierarchical architectures show unexpected performance in energy conversion and storage. To achieve advanced graphene electrode supercapacitors, manipulating the graphene building-blocks into hierarchical nanostructured carbon materials with large electrical double layer capacitance and pseudo-capacitance is a key issue. Here, it is shown that the hierarchically aminated graphitic honeycombs (AGHs) with large surface area for electrical double layer capacitance, tunable surface chemistry for pseudo-capacitance, mediated 3D macropores for ion buffering, and low-resistant pathways for ion diffusion are fabricated for electrochemical capacitive energy storage application through a facile high vacuum promoted thermal expansion and subsequent amination process. In the initial stage of amination (∼200 °C), NH3 reacts with carboxylic acid species to form mainly intermediate amides and amines through nucleophilic substitution. As the temperature increases, the intramolecular dehydration and decarbonylation will take place to generate thermally more stable heterocyclic aromatic moieties such as pyridine, pyrrole, and quaternary type N sites. The AGH exhibits a promising prospect in supercapacitor electrodes with high capacitance (e.g. maximum gravimetric capacitance 207 F g−1 and specific capacitance 0.84 F m−2 at a scan rate of 3 mV s−1) and extraordinary stability (e.g. 97.8% of capacitance retention after 3000 cycles, and 47.8% of capacitance maintaining at a high scan rate of 500 mV s−1 comparing with that at 3 mV s−1). This provides a novel structure platform for catalysis, separation, and drug delivery, which require fast mass transfer through mesopores, reactant reservoirs, and tunable surface chemistry.

 Please wait while we load your content...
Please wait while we load your content...