Catalytic reduction of CO2 into fuels and fine chemicals
Abstract
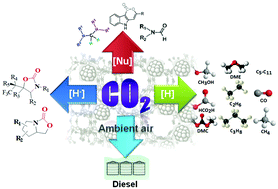
With the progressive increase in atmospheric CO2 over the years and owing to its potential environmental threat, researchers have focused their attention on the fruitful utilization of CO2 into value-added chemicals and feedstocks. Although CO2 conversion reactions are intensively studied through several electrochemical, photochemical and photo-electrochemical pathways, chemical reduction of CO2 is more challenging to achieve due to the involvement of breaking of high energy C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds without any applied potential together with the accomplishment of green chemistry perspectives. Considerable progress in the chemical reduction of CO2 in the presence of reducing agents over homogeneous and heterogeneous catalysts has been achieved over the years. However, this technology has several pitfalls to overcome before it can be utilized in large scale industrial processes. We show here the recent progress in CO2 reduction to essential fuels [CO, CH3OH, CH3CH2OH, HCO2H, CH4, dimethylether (DME), dimethylcarbonate (DMC) and lower hydrocarbons] as well as valuable chemicals via nucleophilic addition reactions. We also emphasize the direct conversion of CO2 from ultra-diluted sources like ambient air as a possible roadmap to solve carbon emission problems from the real world. The entire discussion is divided into two parts where in the first part we summarize several homogeneous catalytic processes involving the nucleophilic addition of CO2, resulting in C–C and C–H bond formation leading to the synthesis of 2-oxazolidinones, aminals, terminal carboxylated products and indolelactone derivatives that are potentially sound for the pharmaceutical industry. Other reduction products, such as methane, methanol, and methoxides, are also listed using Frustrated Lewis Pairs (FLP) as catalysts. The second part extensively highlights heterogeneous catalysts to reduce CO2 with H2. However, significant efforts are still needed to develop active, selective and stable catalysts on a pilot plant scale by judicial consideration of the thermodynamics and kinetics of the reactions. CO2 reduction can proceed over a range of immobilized metallic nanoparticles on inorganic supports (CeO2, Al2O3, TiO2etc.) and nanostructured porous frameworks (zeolites, porous polymers, mesoporous silica). Thus, thorough investigation on the reaction mechanism of the overall process involving different active sites is necessary. Primarily, this review brings together the major advancements made in the CO2 reduction processes together with a focus on the utility and challenges in achieving the activation of the CO2 molecule.
O bonds without any applied potential together with the accomplishment of green chemistry perspectives. Considerable progress in the chemical reduction of CO2 in the presence of reducing agents over homogeneous and heterogeneous catalysts has been achieved over the years. However, this technology has several pitfalls to overcome before it can be utilized in large scale industrial processes. We show here the recent progress in CO2 reduction to essential fuels [CO, CH3OH, CH3CH2OH, HCO2H, CH4, dimethylether (DME), dimethylcarbonate (DMC) and lower hydrocarbons] as well as valuable chemicals via nucleophilic addition reactions. We also emphasize the direct conversion of CO2 from ultra-diluted sources like ambient air as a possible roadmap to solve carbon emission problems from the real world. The entire discussion is divided into two parts where in the first part we summarize several homogeneous catalytic processes involving the nucleophilic addition of CO2, resulting in C–C and C–H bond formation leading to the synthesis of 2-oxazolidinones, aminals, terminal carboxylated products and indolelactone derivatives that are potentially sound for the pharmaceutical industry. Other reduction products, such as methane, methanol, and methoxides, are also listed using Frustrated Lewis Pairs (FLP) as catalysts. The second part extensively highlights heterogeneous catalysts to reduce CO2 with H2. However, significant efforts are still needed to develop active, selective and stable catalysts on a pilot plant scale by judicial consideration of the thermodynamics and kinetics of the reactions. CO2 reduction can proceed over a range of immobilized metallic nanoparticles on inorganic supports (CeO2, Al2O3, TiO2etc.) and nanostructured porous frameworks (zeolites, porous polymers, mesoporous silica). Thus, thorough investigation on the reaction mechanism of the overall process involving different active sites is necessary. Primarily, this review brings together the major advancements made in the CO2 reduction processes together with a focus on the utility and challenges in achieving the activation of the CO2 molecule.


 Please wait while we load your content...
Please wait while we load your content...