Novel β-1,3-d-glucan porous microcapsule enveloped folate-functionalized liposomes as a Trojan horse for facilitated oral tumor-targeted co-delivery of chemotherapeutic drugs and quantum dots†
Abstract
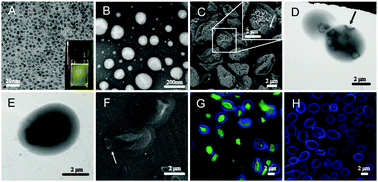
In this study, a new type of β-1,3-D-glucan porous microcapsule (GPM)-enveloped and folate conjugated chitosan-functional liposome (FCL), FCL@GPM, was developed for the potential oral co-delivery of chemotherapeutic drugs and quantum dots (QDs) with facilitated drug absorption and antitumor efficacy. In this dual-particulate system, multiple FCLs serve as the cores for effective loading, folate-mediated tumor-targeting, facilitated intracellular accumulation, and pH-responsive controlled release of chemotherapeutic agents, while a GPM acts as the shell for affording macrophage-mediated tumor selectivity. Gefitinib (GEF) was selected as a chemotherapeutic agent, while acid degradable ZnO QDs were selected due to their dual role as an anticancer agent for synergistic chemotherapy and as a fluorescent probe for potential cancer cellular imaging. The GEF and ZnO QD co-loaded FCL@GPMs (GEF/ZnO-FCL@GPMs) exhibited a prolonged release manner with limited release before uptake by intestinal cells. Furthermore, Peyer's patch uptake, macrophage uptake, cytotoxicity, and biodistribution of FCL@GPMs were tested. In addition, GEF and ZnO QD co-loaded FCLs (GEF/ZnO-FCLs) not only have a tumor acidity responsive release property, but also induce a superior cytotoxicity on cancer cells as compared to GEF. Moreover, a 1.75-fold increase in the bioavailability of GEF delivered from GEF/ZnO-FCL@GPMs as compared to its trademarked drug (Iressa®). As a result, GEF/ZnO-FCL@GPMs exerted a superior antitumor efficacy (1.47-fold) as compared to the trademarked drug in mice. Considered together, the developed FCL@GPMs, combining the unique physicochemical and biological benefits of FCLs and GPMs, possess great potential as an efficient delivery system for the co-delivery of chemotherapeutic agents and quantum dots.


 Please wait while we load your content...
Please wait while we load your content...