Highly effective receptors showing di- vs. monosaccharide preference†
Abstract
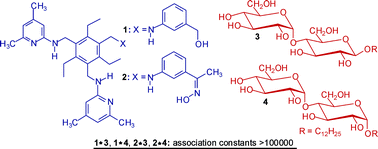
Receptors 1 and 2, incorporating two heterocyclic recognition units as well as oxime- or hydroxymethyl-based hydrogen-bonding sites, were prepared, and their binding properties toward neutral

 Please wait while we load your content...
Please wait while we load your content...