Synthesis, reactivity and complexation studies of N,S exo-heterodisubstituted o-carborane ligands. Carborane as a platform to produce the uncommon bidentate chelating (pyridine)N-C-C-C-S(H) motif†‡§
Abstract
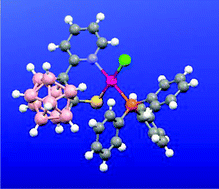
The synthesis of N,S-heterodisubstituted 1-(2′-pyridyl)-2-SR-1,2-closo-C2B10H10 compounds (R = Et, 2; R = iPr, 3) has been accomplished starting from 1-(2′-pyridyl)-1,2-closo-C2B10H11 (1), and their partial deboronation reaction leading to the structurally chiral [7-(2′-pyridyl)-8-SR-7,8-nido-C2B9H10]– derivatives (R = Et, [4]–; R = iPr, [5]–) has been studied.
- This article is part of the themed collection: Collection of articles dedicated to Professor Ken Wade, F.R.S. in celebration of his seventy-fifth birthday

 Please wait while we load your content...
Please wait while we load your content...