Abstract
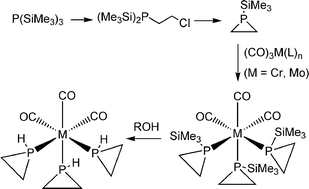
1-Trimethylsilylphosphirane, C2H4PSiMe3, has been prepared on a multi gram scale from P(SiMe3)3via ClCH2CH2P(SiMe3)2. C2H4PSiMe3 is readily susceptible to protonolysis forming the thermally unstable parent phosphirane, C2H4PH, in good yields. Reaction of C2H4PSiMe3 with fac-M (CO)3(CH3CN)3 (M = Cr, Mo) or [Fe(η5-C5H5)(η6-C6H6)](PF6) give rise to fac-M(CO)3(C2H4PSiMe3)3 and [Fe(η5-C5H5)(C2H4PSiMe3)3](PF6) respectively. Protonolysis of the free or coordinated 1-trimethylsilylphosphirane readily causes P–Si cleavage to give rise to the parent C2H4PH or the respective complexes, fac-M(CO)3(C2H4PH)3 and fac-[Fe(η5-C5H5)(C2H4PH)3](PF6) in situ. All new complexes are characterised by analytical and spectroscopic methods and the X-ray crystal structures of fac-Cr(CO)3(C2H4PSiMe3)3 and fac-Mo(CO)3(C2H4PH)3 have also been determined.
- This article is part of the themed collection: Collection of articles dedicated to Professor Ken Wade, F.R.S. in celebration of his seventy-fifth birthday

 Please wait while we load your content...
Please wait while we load your content...