A water-stable Ni(ii) diphosphonate exhibiting water vapor adsorption and water-assisted high proton conductivity†
Abstract
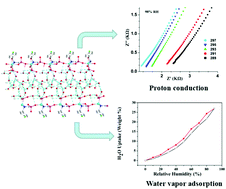
A new nickel(II) phosphonate, [Ni3(HL)2(H2O)10]·4H2O (1), (H4L = 4-F-C6H4CH2N(CH2PO3H2)2) has been synthesized by a hydrothermal method and structurally characterized via X-ray single-crystal diffraction, infrared spectroscopy, elemental analysis and powder X-ray diffraction. 1 exhibits a 1D chain structure formed by the coordination interactions between PO3 groups and metal ions; the chains are further interlinked by [Ni(H2O)6]2+, leading to a supramolecular network. Interestingly, there are helix water channels located in the stacking diagram of 1. To the best of our knowledge, this kind of water channel has not been reported before. The presence of extended hydrogen bonding stabilizes the 3D network and favours proton transfer. 1 shows high proton conductivity with σ = 1.43 × 10−3 S cm−1 at 297 K and 98% RH and a lower activation energy of 0.08 eV. The conductivity is comparable to that of MOF-based materials with efficient proton conducting property. Additionally, the water vapor adsorption of 1 was also investigated.


 Please wait while we load your content...
Please wait while we load your content...