In situ phase transition induced TM–MoC/Mo2C (TM= Fe, Co, Ni, and Cu) heterostructure catalysts for efficient hydrogen evolution†
Abstract
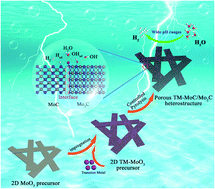
Electrochemical water splitting for large-scale hydrogen production is a potential sustainable method for solving energy and environmental crises. However, the strong bonding strength of hydrogen on the catalyst surface significantly slows down the kinetics of the hydrogen evolution reaction (HER). Herein, we have developed an in situ phase transition strategy to prepare a series of TM–MoC/Mo2C (TM = Fe, Co, Ni, and Cu) heterostructures as catalysts for the HER with the transition metals acting as inducers. The MoC/Mo2C interface and the strong coupling between the TM and MoC/Mo2C enable the reconfiguration of the local electronic environment around the Mo sites, which efficiently balances the binding strength of hydrogen and the charge transfer rate for accelerating the kinetics of the HER. As a result, with adding a 0.5% molar ratio of Co and Mo, the Co–MoC/Mo2C-0.5 catalyst displays a low overpotential at 10 mA cm−2 of 114 mV (0.5 M H2SO4) and 82 mV (1 M KOH) as well as remarkable stability. This work provides an efficient strategy of using transition metal ions in heterojunctions to induce synergistic optimizations in the local electronic structure and molybdenum–hydrogen bond strength to enhance the kinetics of the HER.


 Please wait while we load your content...
Please wait while we load your content...