Coordination chemistry and biology of chelators for the treatment of iron overload disorders
Abstract
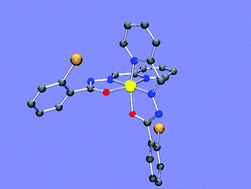
Treatment of the medical condition generally referred to as iron overload through the delivery of
- This article is part of the themed collection: RACI100: Celebrating Australian Chemistry

 Please wait while we load your content...
Please wait while we load your content...