Green transformation of CO2 to ethanol using water and sunlight by the combined effect of naturally abundant red phosphorus and Bi2MoO6†
Abstract
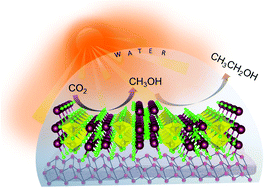
Direct photocatalytic conversion of CO2 to ethanol remains a scientific challenge because of the sluggish kinetics of C–C coupling and complex multielectron transfer processes. To achieve a green transformation of CO2 to C1+ products using naturally abundant sunlight and water requires the smart design of an efficient catalyst by selecting the right combination of atoms either in elemental or in compound form. Herein, we report a composite photocatalyst composed of earth abundant red phosphorus (RP) in nano-sheet morphology decorated with Bi2MoO6 nano-particles. The composite synthesised by a facile ultrasonication method produces 51.8 μmol g−1 h−1 of ethanol from CO2. The ability of RP for the conversion of CO2 to C1 has been altered by the introduction of Bi2MoO6. In situ Diffuse Reflectance Infrared Fourier Transform Spectroscopy (DRIFTS) and Kinetic Isotopic Effect (KIE) analysis shed light on the mechanistic pathway, which propose that the presence of Bi–Mo dual sites play a crucial role in the C–C coupling toward the formation of ethanol. Spectroscopic evidence and isotope labeling experiments suggest that the intermediate OCH3* is the key active species for ethanol formation via self-coupling followed by proton transfer.


 Please wait while we load your content...
Please wait while we load your content...