Copper with an atomic-scale spacing for efficient electrocatalytic co-reduction of carbon dioxide and nitrate to urea†
Abstract
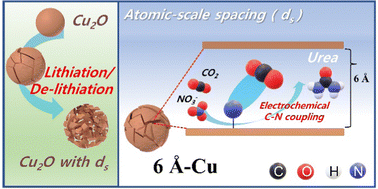
The electrochemical co-reduction of carbon dioxide (CO2) and nitrate (NO3−) to urea via C–N bond coupling is a promising alternative to traditional industrial processes that are intensive in energy consumption and CO2 emission. However, due to the lack of suitable catalysts, the electrochemical process for urea synthesis suffers from low faradaic efficiency, current density, and product yield, which highlights the importance of developing new catalysts that work efficiently toward the co-reduction of CO2 and nitrate NO3− (CR-CO2/NO3−) and the corresponding C–N bond coupling reactions. Here, we report that copper (Cu) with atomic-scale spacings (ds) between copper facets can significantly improve the electrochemical synthesis of urea from CR-CO2/NO3−. We used the lithiation approach to create ds between the copper facets. We prepared four Cu samples with different ds values simply by controlling the degree of lithiation on each sample. Among the four samples, Cu with a ds close to 6 Å achieves a remarkably high urea yield rate of 7541.9 μg h−1 mgcat−1 and a partial current density of 115.25 mA cm−2, substantially greater than those of the bare Cu (urea yield rate of only 444.7 μg h−1 mgcat−1 and urea partial current density of 1.96 mA cm−2) counterpart. Our density functional theory calculations suggest that compared with bare Cu, Cu with a ds of 6.0 Å significantly lowers the energy barrier for C–N coupling, enhancing the C–N bond formation kinetically and thermodynamically and therefore leading to much-improved urea formation from CR-CO2/NO3−.


 Please wait while we load your content...
Please wait while we load your content...