Effect of diphenylphosphinic acid on cesium lead iodide perovskite stability
Abstract
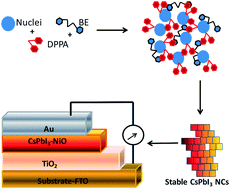
Cesium lead iodide nanocrystals (NCs) are one of the best perovskites to date. The quality of CsPbI3 tends to degrade with time and many researchers have embarked on studies for the improvement of CsPbI3 stability. Herein, CsPbI3 nanoparticles were synthesized via a colloidal approach by varying the capping agent and the coordinating solvent. The effect of a phosphinic ligand on the synthesis of CsPbI3 was assessed with the aim of getting new properties and/or improving its existing properties. The stability of the synthesized CsPbI3 perovskites was evaluated through photoluminescence emission over time. A nearly cubic phase of CsPbI3 was obtained through synthesis with diphenylphosphinic acid (DPPA) in benzyl ether (BE). Smaller nanocubes with an average size of 14 nm were prepared using DPPA in BE. The emission intensity lasted beyond 6 days with only 40% emission lost compared to that of materials prepared using a mixture of solvents and the conventional method. In order to illustrate possible applications, the NCs were used in a simple solar cell device with the configuration substrate-FTO-TiO2-CsPbI3/NiO-Au. A power conversion efficiency and a fill factor of 2.0% and 38%, respectively, were obtained from a 2,2′,7,7′-tetrakis-(N,N-di-p-methoxyphenylamine)-9,9′-spirobifluorene (spiro-MeOTAD)-free solar device which was made of the more stable synthesized CsPbI3.


 Please wait while we load your content...
Please wait while we load your content...