Thioester hydrolysis reactivity of zinc hydroxide complexes: investigating reactivity relevant to glyoxalase II enzymes†
Abstract
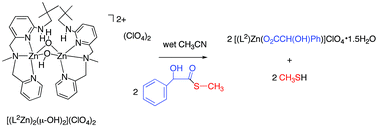
A recently reported binuclear zinc hydroxide complex [(L1Zn2)(µ-OH)](ClO4)2 (1, L1 = 2,6-bis[(bis(2-pyridylmethyl)amino)methyl]-4-methylphenolate monoanion) containing a single bridging hydroxide was examined for thioester hydrolysis reactivity. Treatment of 1 with hydroxyphenylthioacetic acid S-methyl ester in dry CD3CN results in no reaction after ∼65 h at 45(1) °C. Binuclear zinc hydroxide complexes of the N-methyl-N-((6-neopentylamino-2-pyridyl)methyl)-N-((2-pyridyl)methyl)amine (L2) and N-methyl-N-((6-neopentylamino-2-pyridyl)methyl)-N-((2-pyridyl)ethyl)amine (L3) chelate ligands were prepared by treatment of each ligand with molar equivalent amounts of Zn(ClO4)2·6H2O and KOH in methanol. These complexes, [(L2Zn)2(µ-OH)2](ClO4)2 (2) and [(L3Zn)2(µ-OH)2](ClO4)2 (3), which have been structurally characterized by X-ray crystallography, behave as 1 : 1 electrolytes in acetonitrile, indicating that the binuclear cations dissociate into monomeric zinc hydroxide species in solution. Treatment of 2 or 3 with one equivalent of hydroxyphenylthioacetic acid S-methyl ester per zinc center in acetonitrile results in the formation of a zinc α-hydroxycarboxylate complex, [(L2)Zn(O2CCH(OH)Ph)]ClO4·1.5H2O (4) or [(L3)Zn(O2CCH(OH)Ph)]ClO4·1.5H2O (5), and CH3SH. These reactions, to our knowledge, are the first reported examples of thioester hydrolysis mediated by zinc hydroxide complexes. The results of this study suggest that a terminal Zn–OH moiety may be required for hydrolysis reactivity with a thioester substrate.

 Please wait while we load your content...
Please wait while we load your content...