DRIFTS study of a Ce–W mixed oxide catalyst for the selective catalytic reduction of NOx with NH3
Abstract
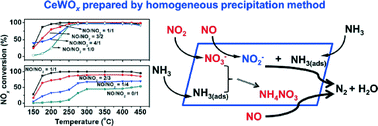
A systematic study has been conducted on the reactivity of the selective catalytic reduction of NOx with NH3 (NH3-SCR) in a wide range of NO/NO2 feed ratios (from 0 : 1 to 1 : 0) over a CeWOx catalyst prepared by a homogeneous precipitation method. By using in situ diffuse reflectance infrared Fourier transform spectroscopy (in situ DRIFTS), the roles of NO and NO2 at low temperatures during the fast SCR reaction have been revealed. NO2 adsorption results in the formation of surface nitrates, which participate in the NH3-SCR reaction through two pathways: one path where the nitrates react with NH3 to form ammonium nitrate (NH4NO3), then NO reduces NH4NO3 below its melting point to form N2 (the NH4NO3 path); the other path where the surface nitrates are reduced by NO to form active nitrite species that further react with NH3 to produce N2 (the nitrate path). “The NH4NO3 path” and “the nitrate path” contributed simultaneously to the standard and the fast SCR reaction at low temperatures. Both the surface nitrates and NO in the gas phase were suggested to be necessary for the excellent performance of the fast SCR reaction over the CeWOx catalyst.

 Please wait while we load your content...
Please wait while we load your content...