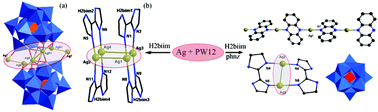
Two unusual α-Keggin-based compounds with multinuclear silver clusters, {[Ag7(H2biim)5][PW11O39]}·Cl·H3O (1) and {[Ag2(H2biim)2][Ag(phnz)](PW12O40)}·H2O (2) (H2biim = 2,2′-biimidazole, phnz = phenazine), have been hydrothermally synthesized and structurally characterized by elemental analyses, IR spectra, thermogravimetric analysis and X-ray crystallography. Compound 1 displays a 2D network featuring dimerized monolacunary Keggin anions {PW11O39}2 which are connected together through pentanuclear silver clusters. Interestingly, besides {Ag5}5+ clusters, there are another kinds of argentophilic {Ag4}4+ clusters coexisting in compound 1. Compound 2 consists of four subunits: 1D cationic chains [Ag(phnz)]+, dinuclear silver-H2biim units [Ag2(H2biim)2]2+, discrete polyanions {PW12O40}3− and water molecules, which are further joined by hydrogen bonds to form a 3D supramolecular structure. The differences in the structures of compounds 1 and 2 indicate that the introduction of the secondary organic ligand plays a key role in determining the final structure. The electrochemical, electrocatalytic behaviors and luminescent properties of compounds 1 and 2 have also been investigated.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...