Heterologous expression and characterization of an endoglucanase from Lactobacillus plantarum dy-1†
Abstract
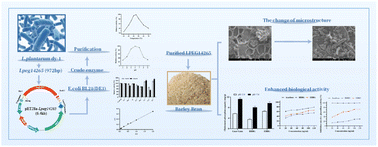
The structure and function of β-glucan in barley have been reported to change significantly after fermentation with Lactobacillus plantarum dy-1, but little information is available to explain this phenomenon. The Carbohydrate-Active enZYmes database revealed that L. plantarum dy-1 encodes 158 types of glycosidic hydrolases, among which we have identified an endoglucanase. Therefore, we conducted a heterologous expression of this endoglucanase gene, namely Lpeg14265. The pH of 6.0 and the temperature of 60 °C were optimal for LPEG14265. The physiological activities of β-glucan, such as the capacity to adsorb cholesterol or to block α-amylase and α-glucosidase, increased as a result of enzymatic hydrolysis of LPEG14265, which also caused a significant change in the microstructure of barley bran. Based on these findings, it was concluded that barley bran, a by-product of agriculture, may be processed with LPEG14265 to reveal its potential value, which could have applications in the brewing and feed industries, among others.


 Please wait while we load your content...
Please wait while we load your content...