Heterocyclic ring scaffolds as small-molecule cholesterol absorption inhibitors
Abstract
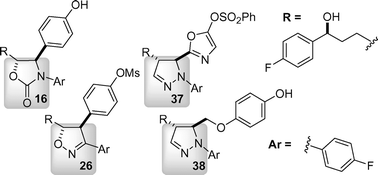
Enantio- and diastereoselective syntheses of a substituted oxazolidinone, isoxazoline and pyrazoline as β-lactam surrogates are described. The substituted heterocycles were designed to incorporate side chains closely resembling those found in the β-lactam cholesterol absorption inhibitor ezetimibe (1). Additionally, the in vitro inhibitory efficacy of the novel compounds as cholesterol absorption inhibitors is reported using a brush border membrane vesicle assay.

 Please wait while we load your content...
Please wait while we load your content...