Synthetic strategies to chiral organosulfur donors related to bis(ethylenedithio)tetrathiafulvalene
Abstract
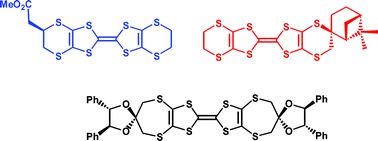
Syntheses of enantiopure organosulfur donors by three different strategies requiring only four–six steps are reported. The key step involves either double substitution of an enantiopure cyclic sulfate ester by a dithiolate, attachment of a chiral
- This article is part of the themed collection: In memory of Peter Day

 Please wait while we load your content...
Please wait while we load your content...