Reactions of 1-haloadamantanes and ethylmercury chloride with nitronate anions by the SRN1 mechanism
Abstract
Secondary or tertiary nitro compounds can be easily obtained by photostimulated reaction of the anions corresponding to primary or secondary nitroalkanes with either 1-iodoadamantane (1-AdI) or ethylmercury chloride (EtHgCl) by the SRN1 mechanism. Only 1-AdI requires the presence of the enolate anion of acetone as an entrainment reagent. The procedure works well with the nitroanions derived from 1-nitropentane, nitroethane or 6-nitrohex-1-ene, but with 2-nitropropane ion the outcome depends on the substrate and solvent.
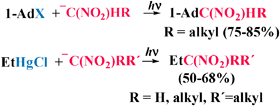

 Please wait while we load your content...
Please wait while we load your content...