Acenaphthylene-fused ullazines: fluorescent π-extended monopyrroles with tunable electronic gaps†
Abstract
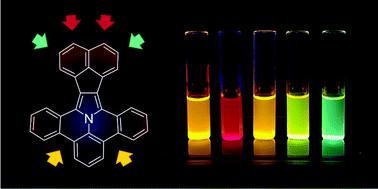
π-Extended dibenzoullazines containing an acenaphthylene subunit were designed and synthesized. Two different synthetic strategies were employed: route A, based on Pd-catalyzed cyclodehydrohalogenation of α,α-disubstituted N-arylpyrroles, and route B, using a dipolar 1,3-cycloaddition reaction of azomethine ylides (PAMYs) to functionalized acenaphthylenes. Molecules of the resulting ullazines are almost flat, leading to strong π–π interactions in the solid state. The new ullazines are highly fluorescent (with a quantum yield of up to 0.89 for the naphthalimide-fused system), and show moderate solvatochromism with no fluorescence quenching in polar solvents. Stepwise two-electron oxidation of the ullazines is possible, yielding reversibly the corresponding ullazine radical cations and dications. Edge expansion of the ullazine core with methylene bridges is additionally shown to produce an ullazine analogue containing two seven-membered rings in its structure, which is characterized by axial chirality and can be resolved into enantiomers.


 Please wait while we load your content...
Please wait while we load your content...