Partially H-bonded covalent organic frameworks for photocatalytic hydrogen evolution†
Abstract
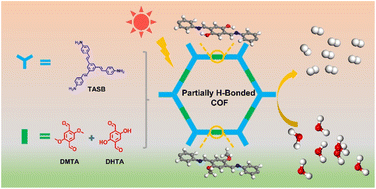
Photocatalysis is considered to be one of the most effective methods to solve the global energy problem. Therefore, it is essential to clarify the principles of the design and synthesis of highly effective photocatalysts. In this paper, we report a series of covalent organic frameworks (denoted as COF-960-n) with different degrees of intralayer hydrogen-bonding interactions via a multivariate synthetic strategy. Although the resultant COFs have similar chemical structures, good crystallization, and high surface area, the overall photocatalytic hydrogen evolution changed significantly due to the different ratios of hydroxyl to methoxy groups. COF-960-5 exhibited the best charge transfer rate and the lowest electron–hole recombination rate, resulting in the best hydrogen evolution rate (678 μmol g−1 h−1) under visible light irradiation. Moreover, the hydrogen-bonding interactions help to enhance the stability of COFs during photocatalysis. This study demonstrates that partial H-bonding can lead to a better photocatalytic H2 evolution, providing insight into the further design of efficient COF-based photocatalysts for H2 evolution.
- This article is part of the themed collections: Journal of Materials Chemistry A Emerging Investigators and #MyFirstJMCA


 Please wait while we load your content...
Please wait while we load your content...