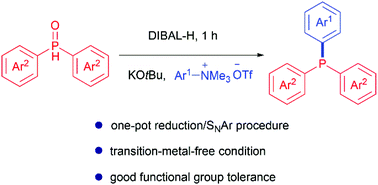
Lei Zhang, Chengyu Liu, Lei Yang, Liming Cao, Chaoming Liang, Maolin Sun, Yueyue Ma, Ruihua Cheng and Jinxing Ye
Org. Biomol. Chem., 2022,20, 3897-3901
DOI:
10.1039/D2OB00547F,
Communication