Nanostructured morphology control and phase transition of zeolitic imidazolate frameworks as an ultra-high performance adsorbent for water purification†
Abstract
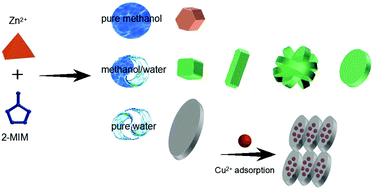
The design of materials that target heavy metal ions with fast and highly effective adsorption performance is imperative for the removal of heavy metal contaminants from water ecosystems. Inspired by the “function follows form” principle, herein, we developed an approach to control the morphologies and phases of zeolitic imidazolate framework-based nanomaterials (denoted as ZIF-8-x, where x represents the methanol/water ratio) to achieve an ultra-high heavy metal adsorption property. By tuning the solution conditions, the transformation of the morphology of various obtained nanocrystals from a typical rhombic dodecahedron to leaf shape, accompanied by a phase transition from the ZIF-8 to the ZIF-L phase and an increase in amorphous regions, was achieved; this benefited the adsorption interaction between heavy metal ions and 2-methylimidazole binding sites that enhanced the adsorption capacities. The optimized ZIF-8-x of ZIF-8-0 (synthesized in pure water) with leaf-like morphology and the ZIF-L phase endows the prepared adsorbent with extra imino groups for the adsorption of heavy metal ions, which exhibits highest adsorption capacity towards Cu(II) (∼1457.87 mg g−1) and quickly achieves adsorption equilibrium in 5 min. Our results suggest that enhanced adsorption can be achieved by engineering the morphology and phase of nanocrystals, and the as-prepared ZIF-8-0 can be a promising candidate for the purification of water.
- This article is part of the themed collection: 2019 Inorganic Chemistry Frontiers HOT articles


 Please wait while we load your content...
Please wait while we load your content...