Artificial supramolecular light-harvesting systems based on a pyrene derivative for photochemical catalysis†
Abstract
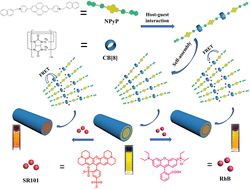
Two efficient energy transfer systems were fabricated in an aqueous solution using a water-soluble naphthalene-modified pyrene derivative (NPyP), cucurbit[8]uril (CB[8]), and sulforhodamine 101 (SR101) or rhodamine B (RhB). The naphthalene units of NPyP can be encapsulated by CB[8] based on host–guest interactions, further forming rod-like nanostructures. The NPyP-CB[8] assemblies could avoid the aggregation-caused quenching (ACQ) effect of the pyrene derivative in an aqueous solution and exhibit strong fluorescence emission. Significantly, after the addition of RhB or SR101, an efficient Förster resonance energy transfer process occurred from NPyP-CB[8] to RhB or SR101, respectively. Furthermore, to best utilize the harvested energy, photochemical catalysis using NPyP-CB[8] + RhB or NPyP-CB[8] + SR101 was performed for high-yield dehalogenation of α-bromoacetophenone in an aqueous medium.


 Please wait while we load your content...
Please wait while we load your content...