The role of oxygen vacancies in improving the performance of CoO as a bifunctional cathode catalyst for rechargeable Li–O2 batteries†
Abstract
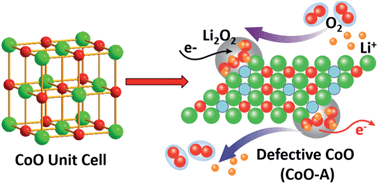
The design and facile synthesis of noble metal-free efficient cathode catalysts to accelerate the sluggish kinetics of both oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) is still a big challenge for lithium–air batteries. In this study, oxygen vacancy-bearing CoO (CoO-A) has been successfully synthesized through a simple calcination of Co(Ac)2·4H2O in Ar, and the oxygen vacancies have been confirmed by Raman spectroscopy, HRTEM, X-ray photoelectron spectroscopy (XPS) and positron annihilation lifetime spectroscopy (PALS). In comparison with defect-free CoO-N, which is derived from the decomposition of Co(NO3)2·6H2O, an oxygen-deficient CoO-A based cathode shows much higher cycling stability, higher rate capability, higher coulombic efficiency, and lower charge–discharge overpotential. The enhanced performances of the CoO-A based cathode can be largely attributed to the synergetic effect of CoO itself and oxygen vacancies on the promotion of both ORR and OER. CoO provides catalytic activity for ORR, and at the same time the oxygen vacancies not only facilitate the electron and Li+ migration but also act as active sites binding to O2 and Li2O2, which accelerates the OER process. Furthermore, the formation and decomposition of Li2O2 during discharge–charge cycles have also been studied and the results indicate that CoO-A shows a high catalytic activity in the decomposition of Li2O2.

 Please wait while we load your content...
Please wait while we load your content...