Facile template free approach for the large-scale solid phase synthesis of nanocrystalline XIn2S4 (X = Cd/Zn) and its photocatalytic performance for H2 evolution†
Abstract
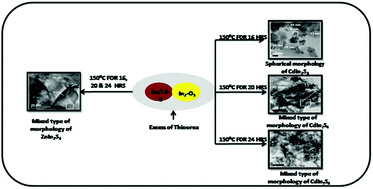
In the present study, we have demonstrated a unique approach for the synthesis of nanostructured ternary metal sulphides, XIn2S4 (X = Cd, Zn), using a simple, template free and low temperature solid phase reaction. A stoichiometric amount of precursors including CdO/ZnO, In2O3 and thiourea were mixed homogeneously and heated at 150 °C for different time intervals to produce nanostructured XIn2S4. Various molar ratios of the precursors were tried and among the different ratios, the molar ratio of 1 : 1 : 8 was found to be optimum for the growth of phase pure nano crystalline XIn2S4. X-ray analysis confirms the formation of cubic spinel Cadmium Indium Sulfide (CdIn2S4) and hexagonal Zinc Indium Sulfide (ZnIn2S4). HRTEM analysis confirms the formation of nanostructures of XIn2S4 with spherical and rod like mixed morphology. The band gap of the as synthesized materials is within the visible region i.e. 2.25 and 2.6 eV for CdIn2S4 and ZnIn2S4, respectively. Therefore, the activity of both the photocatalyts was tested for hydrogen generation by H2S and H2O splitting under natural solar light. The ZnIn2S4 photocatalyst was found to be more active for both H2S and H2O splitting reactions with H2 evolution rates of 6994 μmol h−1 g−1 and 243 μmol g−1, respectively, whereas for CdIn2S4 the rates were found to be 6128 μmol h−1 g−1 and 128.4 μmol g−1, respectively. The H2 evolution rate by H2S splitting was found to be much higher than various previously reported nanostructured photocatalysts. The simple, environmentally friendly and cost effective method reported herein can be applied for the large scale synthesis of XIn2S4 nanoparticles, which is the novelty of the present work.


 Please wait while we load your content...
Please wait while we load your content...